MENU

สอบ CU-ATS คืออะไร ?
Chulalongkorn University Aptitude Test for Science หรือ CU-ATS คือ ข้อสอบความถนัดในด้านวิทยาศาสตร์ของทางมหาวิทยาลัยจุฬาลงกรณ์ คะแนนรวม 1,600 คะแนน ประกอบด้วย 2 วิชา ได้แก่ เคมี และ ฟิสิกส์
CU-ATS สอบอะไรบ้าง ?
สอบ CU-ATS คือ ประกอบด้วย 2 วิชา ได้แก่ CU-ATS Physics 30 ข้อ ใช้เวลาสอบ 60 นาที คะแนนเต็ม 800 และ CU ATS Chemistry มี 55 ข้อ ใช้เวลาสอบ 60 นาที คะแนนเต็ม 800
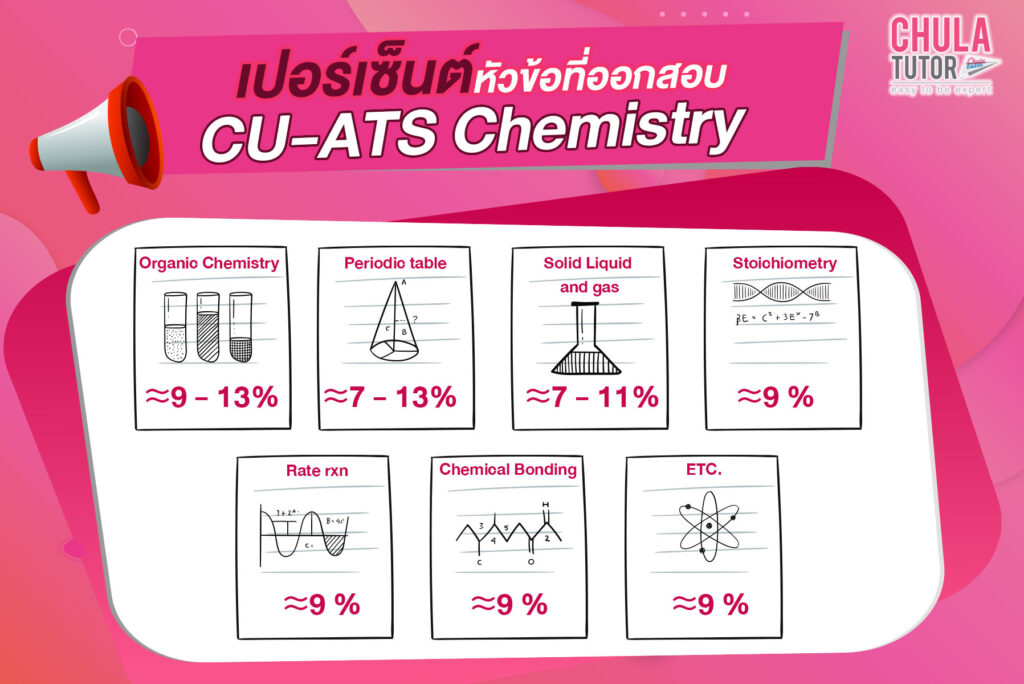
ข้อสอบ CU-ATS Chemistry 55 ข้อ 60 นาที 800 คะแนน
ข้อสอบ CU ATS Chemistry มีข้อสอบทั้งหมด 55 ข้อ เวลาสอบ 60 นาที คะแนนเต็ม 800 ข้อสอบออกเรื่องหลักๆ คือ Mechanics , Electricity and Magnetism, Waves ,Heat , Kinetic ,Theory and Thermodynamics , Modern Physics และ Miscellaneous
เทคนิคทำข้อสอบ พาร์ท Chemistry
ข้อสอบส่วนพาร์ทเคมี จะออก 5 เรื่องหลักๆ คือ
- Chemical bonding ข้อสอบจะออกประมาณ 20% ของข้อสอบ
- Solids Liquids and Gases บทนี้ข้อสอบจะออกทั้งคำนวณ และ ทฤษฎี
- Acids and Base ข้อสอบจะออกทั้งคำนวณ และ ทฤษฎี โดยมักจะออกรวมกับเรื่องอื่นๆด้วย
- Organic Chemistry บทนี้จะเป็นบทที่มี ทฤษฎี เยอะมากแต่เป็นที่ค่อนข้างง่าย น้องๆควรเก็บคะแนนบทนี้ให้ได้นะครับ
- Electrochemistry ข้อสอบจะออกทั้งคำนวณ และ ทฤษฎี
สรุป พาร์ท Chemistry ข้อสอบจะออกทั้งคำนวณ และ ทฤษฎี น้องๆ ต้องแม่นเรื่องหลักการณ์ ส่วนโจทย์ข้อสอบมักจะมีทั้งแบบพื้นฐานเคมีทั่วไปและแบบประยุกต์ ซึ่งตัวข้อสอบออกจะมีทั้งแบบที่ง่ายและยากผสมกัน น้องๆจะต้องแบ่งเวลาข้อสอบให้ดี

ข้อสอบ CU-ATS Physics 30 ข้อ 60 นาที 800 คะแนน
ข้อสอบ CU ATS Physics มีข้อสอบทั้งหมด 30 ข้อ เวลาสอบ 60 นาที คะแนนเต็ม 800 ข้อสอบออกเรื่องหลักๆ คือ
- Structure of Matter
- Atom theory and structure
- Molecular structure
- Bonding, States of Matter
- Gases , Solutions
- Reaction Types
- Acids and bases
- Oxidation-reduction
- Stoichiometry
- Mole concept
- Chemical equations
- Equilibrium and Reaction Rates
- Thermochemistry
- Descriptive Chemistry
เทคนิคทำข้อสอบ พาร์ท Physics
ข้อสอบพาร์ท ฟิสิกส์ จะออก 5 เรื่องหลักๆ คือ
- Newton’s laws of motion
- Mechanics: Work & Evergy
- EM fields/forces
- Basic of waves
- Energy levels
ข้อสอบพาร์ท Physics มีทั้งหมด 30 ข้อ น้องควรใช้เวลาเฉลี่ยข้อหนึ่งไม่เกิน 2 นาที เพราะการทำข้อสอบให้เร็วถือเป็นหัวใจสำคัญของการทำคะแนนในพาร์ทนี้
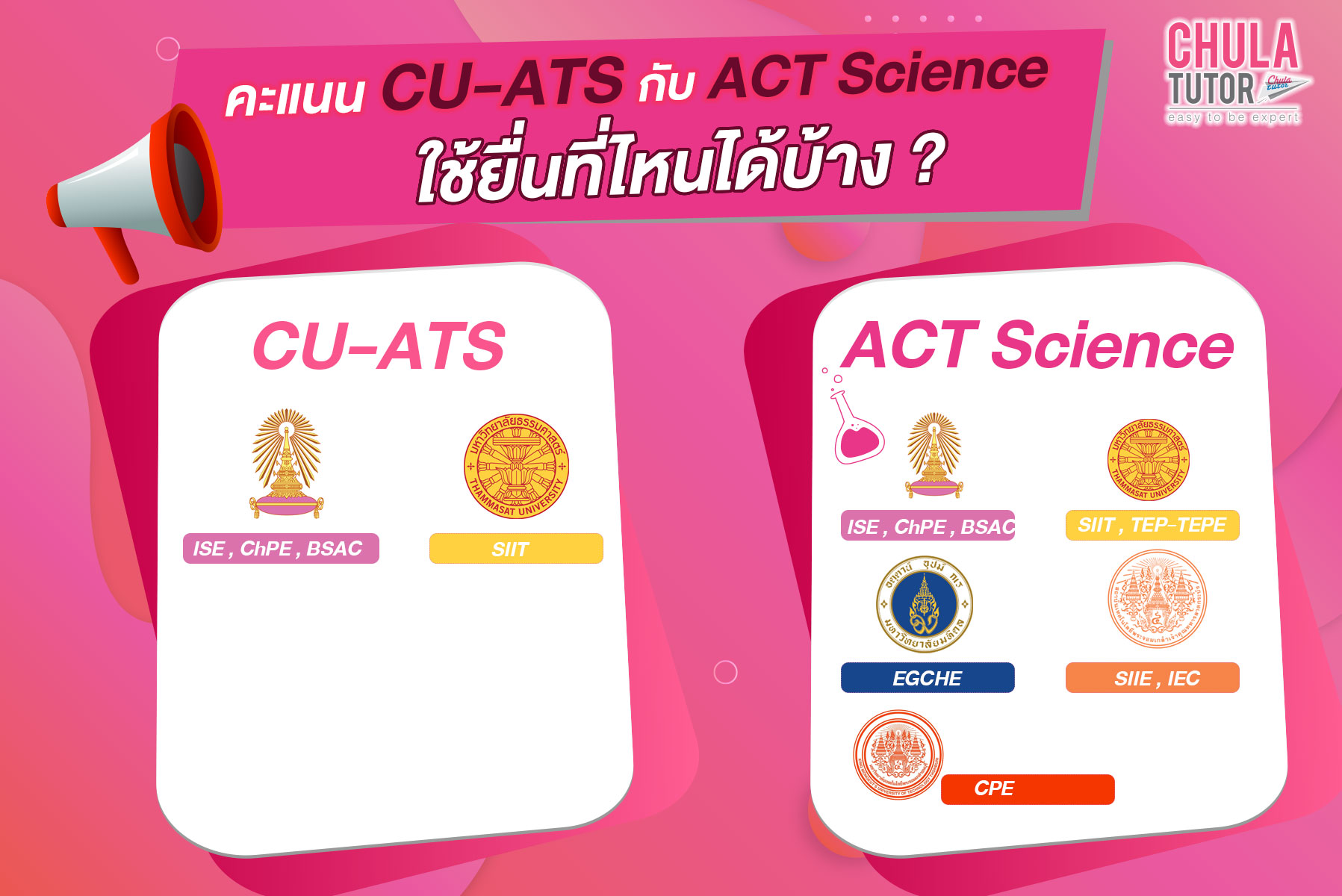
คะแนน CU-ATS ยื่นอะไรได้บ้าง ?
ISE : คณะวิศวกรรมศาสตร์ จุฬาลงกรณ์มหาวิทยาลัย (อินเตอร์) คะแนนรวมวิชาเคมีและฟิสิกส์ ไม่น้อยกว่า 800 คะแนน
ISE : คณะวิศวกรรมศาสตร์ จุฬาลงกรณ์มหาวิทยาลัย (อินเตอร์) สาขาวิชาเคมีวิศวกรรมและกระบวนการ คะแนนรวมวิชาเคมีและฟิสิกส์ ไม่น้อยกว่า 800 คะแนน
BSAC : คณะวิทยาศาสตร์ สาขาวิชาเคมีประยุกต์ จุฬาลงกรณ์มหาวิทยาลัย (อินเตอร์) รับคะแนน Chemistry โดยมีเกณฑ์คะแนนไม่น้อยกว่า 380 คะแนน
ChPE : Chemical and Process Engineering
KMITL’S AEC : วิศวกรรมการบินและนักบินพาณิชย์ สถาบันเทคโนโลยีพระจอมเกล้าเจ้าคุณทหารลาดกระบัง รับคะแนน โดยไม่กำหนดคะแนนขั้นต่ำ
วิศวะอินเตอร์จุฬา มีสาขาอะไรบ้าง ?
วิศวกรรมศาสตร์ นานาชาติ (ISE) มีสาขาเรียนทั้งหมด 5 สาขา ได้แก่
สาขาวิชาวิศวกรรมอากาศยาน
สาขาวิชาวิศวกรรมการออกแบบและการผลิตยานยนต์
สาขาวิศวกรรมนาโน
สาขาวิชาวิศวกรรมสารสนเทศและการสื่อสาร
สาขาวิชาวิศวกรรมหุ่นยนต์และปัญญาประดิษฐ์
วิศวกรรมศาสตร์ นานาชาติ (ChPE) มี 1 สาขา คือ
สาขาวิชาเคมีวิศวกรรมและกระบวนการ
วิธีสมัครสอบ CU-ATS
รอบสอบในแต่ละปี จะมีการจัดสอบประมาณ 4 รอบ ครอบคลุมทั้งช่วงต้นปี กลางปี และปลายปี
โดยน้องสามารถเลือกได้ว่าจะสอบแบบสอบกับกระดาษ หรือ จะเลือกสอบแบบ E-Testing (สอบกับคอมพิวเตอร์) ซึ่งโดยส่วนใหญ่แล้วการสอบทั้ง 2 แบบ มักจะเป็นวันเดียวกัน ผู้สอบจึงต้องตัดสินใจเลือกว่าต้องการสอบแบบ paper หรือแบบ computer โดยสามารถอัพเดตรอบสอบได้ที่ http://atc.chula.ac.th/Main/ats_th/
ขั้นตอนสมัครสอบ CU ATS
- เข้าเว็บไซต์ศูนย์ทดสอบทางวิชาการแห่งจุฬาลงกรณ์มหาวิทยาลัย http://www.atc.chula.ac.th
- เลือกเมนู “เข้าสู่ระบบลงทะเบียนออนไลน์” หากเคยลงทะเบียนแล้วให้กรอกข้อมูล log in ได้เลย แต่หากยังไม่เคยลงทะเบียนมาก่อน ให้เลือกเมนู “ลงทะเบียนใหม่”
- กรอกข้อมูลส่วนตัวให้ถูกต้องและครบถ้วน โดยเฉพาะช่องที่มีเครื่องหมาย * หมายถึงจำเป็นต้องกรอกข้อมูลให้ครบ
- หากอยู่ในช่วงที่กำลังเปิดรับสมัครสอบ ให้เราเลือกรอบสอบ และวิชาสอบที่ต้องการได้เลย
- พิมพ์ใบชำระเงินออกมา นำไปชำระเงินตามช่องทางที่ระบุไว้
ค่าสอบ CU-ATS
ค่าสมัครสอบ CU-ATS (PBT) 1,000 บาท
ค่าสมัครสอบ CU-ATS (CBT) 2,600 บาท
ตารางสอบ CU-ATS Tests 2025
ทางศูนย์ทดสอบจุฬาฯ จะจัดสอบ CU-ATS Tests ปีละ 4-5 ครั้ง คือจะมีสอบในช่วงเดือน กุมภาพันธ์ , มีนาคม , กรกฎาคม , และ ธันวาคม โดยปกติจะสอบช่วงเวลา 13:00 – 15:00 น.ของทุกรอบในการสอบ และสำหรับน้อง คนไหนที่ต้องการยื่นคะแนนรอบ Early ควรมีคะแนนก่อนเดือนธันวาคม
ตารางสอบ CU ATS 2568
| Test Dates | เวลา | ช่วงรับสมัคร |
| 16 มี.ค 68 | 13:00 – 15:00 | 3 – 9 มี.ค. 68 |
| 6 ก.ค. 68 | 13:00 – 15:00 | 23 – 29 มิ.ย. 68 |
| 5 ต.ค. 68 | 13:00 – 15:00 | 22 – 28 ก.ย. 68 |
| 9 พ.ย. 68 | 13:00 – 15:00 | 27 ต.ค. – 2 พ.ย. 68 |
| 14 ธ.ค. 68 | 13:00 – 15:00 | 1 – 7 ธ.ค. 68 |

รีวิว คอร์ส ติว CU-ATS ตัวต่อตัว

รีวิวข้อสอบ CU-ATS Chemistry
ภาพรวมของข้อสอบ CU ATS เคมี
ข้อสอบ CU-ATS Chemistry เป็นข้อสอบที่ใช้วัดความเข้าใจในเนื้อหาวิชาเคมีระดับมัธยมศึกษาตอนปลาย โดยเน้นทั้ง ความรู้เชิงทฤษฎี และ การคำนวณทางเคมี ข้อสอบเป็น แบบปรนัย (Multiple Choice Questions – MCQs) มีตัวเลือก 5 ข้อ และระดับความยากตั้งแต่พื้นฐานจนถึงขั้นสูง
โครงสร้างข้อสอบ & ตัวอย่างแนวข้อสอบ
1. ไฟฟ้าเคมี (Electrochemistry) & ปฏิกิริยารีดอกซ์ (Redox Reactions)
- วิเคราะห์ ศักย์ไฟฟ้ามาตรฐาน (E°) ของเซลล์
- หาสารที่เป็น ตัวออกซิไดซ์ (Oxidizing Agent) และตัวรีดิวซ์ (Reducing Agent)
- เช่น ต้องเลือกสารที่มีค่า E° สูงสุดเป็นตัวออกซิไดซ์ที่แรงที่สุด
2. เคมีฟิสิกส์ (Physical Chemistry): ความดันไอ & แรงตึงผิว
- เปรียบเทียบ ความดันไอ (Vapor Pressure) กับอุณหภูมิ
- หาว่าสารใดมี แรงตึงผิวต่ำสุด เช่น Ether, น้ำ (Water), ปรอท (Mercury)
3. กฎของแก๊ส & อุณหพลศาสตร์ (Gas Laws & Thermodynamics)
- ใช้ กฎของแก๊สในอุดมคติ (PV = nRT) และ กฎรวมของแก๊ส
- เช่น คำนวณปริมาตรของลูกโป่งเมื่ออุณหภูมิและความดันเปลี่ยนแปลง
4. เคมีกรด-เบส & การคำนวณค่า pH
- หาค่า pH ของสารละลายเกลือเบสิก จากค่า Ka1 และ Ka2
- ใช้ความสัมพันธ์ระหว่าง Ka, Kb และ pH
5. สมบัติของสารละลาย (Colligative Properties)
- วิเคราะห์ จุดเดือดของสารละลาย โดยใช้หลักการ Boiling Point Elevation
- เปรียบเทียบสารเช่น Lactose, Galactose, Ribose ว่าตัวใดมีจุดเดือดสูงสุด
6. สมดุลเคมี (Chemical Equilibrium)
- ใช้ ICE Table คำนวณหาความเข้มข้นของสารที่สมดุล
- เช่น ให้ค่า K และความเข้มข้นเริ่มต้นของสาร A และ B หา [B] ที่สมดุล
7. เคมีอินทรีย์ (Organic Chemistry) & รูปร่างโมเลกุล
- วิเคราะห์ โครงสร้างโมเลกุล และรูปทรงของ Carbon (C) ใน DMAU
- เช่น ค้นหาว่าโครงสร้างหลักของโมเลกุลเป็น Tetrahedral หรือ Trigonal Planar
8. ชีวเคมี & Benedict’s Test
- ทดสอบ น้ำตาลรีดิวซ์ ด้วย Benedict’s Test
- ตัวอย่าง: สารที่ไม่ทำปฏิกิริยากับ Benedict’s Solution แต่เปลี่ยนเป็น ตะกอนสีแดงอิฐ หลังไฮโดรไลซ์ เช่น แป้ง (Tapioca Flour)
9. ตารางธาตุ & โครงสร้างอะตอม
- หาธาตุจาก มวลอะตอมสัมพัทธ์ และจำนวนโปรตอน/นิวตรอน
- เช่น ธาตุ X มี มวลอะตอมมากกว่า Tritium 14 เท่า และมีนิวตรอนมากกว่า Deuterium 22 เท่า
10. เคมีเชิงซ้อน (Coordination Chemistry) & อิเล็กตรอนของโลหะทรานซิชัน
- หาค่าการจัดเรียงอิเล็กตรอนของ Cobalt (Co) ในสารประกอบ [Co(NH₃)₄SO₄]NO₃
11. ธาตุกัมมันตรังสี & กฎของครึ่งชีวิต (Radioactivity & Half-life Calculations)
- ใช้สมการ Half-life หาว่าธาตุ Z ที่เหลืออยู่หลังจากเวลาที่กำหนดคืออะไร
12. การแพร่ของแก๊ส & อัตราการรั่วไหล (Gas Diffusion & Leakage Rate)
- เปรียบเทียบว่า แก๊สใดรั่วออกเร็วที่สุด โดยใช้ มวลโมลาร์
- เช่น H₂, He, N₂, O₂, Ne
13. อุณหพลศาสตร์ & ศูนย์องศาสัมบูรณ์ (Absolute Zero & Entropy)
- วิเคราะห์ความเข้าใจผิดเกี่ยวกับ Entropy ที่ศูนย์องศาสัมบูรณ์
14. ชีวเคมี & โครงสร้างของ DNA และ RNA
- ความแตกต่างระหว่าง DNA กับ RNA เช่น น้ำตาล, เบส, พันธะไฮโดรเจน
15. สูตรโมเลกุล & สารประกอบไฮโดรคาร์บอน
- วิเคราะห์ว่าโมเลกุลใดมีสูตร C₇H₁₀
16. บัฟเฟอร์ (Buffer Solutions)
- คำนวณว่าสารคู่ใดสามารถเป็น บัฟเฟอร์ ได้
17. สูตรเคมีของสารสำคัญ
18. อัตราการเกิดปฏิกิริยา
เทคนิคเตรียมสอบ CU ATS Chemistry
- อ่านทฤษฎีให้แม่น – เข้าใจ สมดุลเคมี, กรด-เบส, ไฟฟ้าเคมี, อุณหพลศาสตร์
- ฝึกคำนวณให้คล่อง – เน้น กฎของแก๊ส, pH, Ksp, Ka, Kb
- ทบทวนเคมีอินทรีย์ – โครงสร้างโมเลกุล, ไฮบริดไดเซชัน, กลุ่มฟังก์ชัน
- ฝึกทำข้อสอบเก่า – ทำ CU-ATS Chemistry ปีเก่าๆ หรือ SAT Chemistry
- จำสูตรสำคัญ – เช่น PV = nRT, pH = -log[H⁺], อัตราการเกิดปฏิกิริยา
รีวิวข้อสอบ CU-ATS Physics
ภาพรวมของข้อสอบ CU-ATS ฟิสิกส์
ข้อสอบ CU-ATS Physics เป็นข้อสอบที่ออกแบบมาเพื่อวัดความเข้าใจในฟิสิกส์ระดับมัธยมศึกษาตอนปลาย โดยเน้นทั้ง ความรู้เชิงทฤษฎี และ การคำนวณเชิงกลศาสตร์ เป็นหลัก ข้อสอบเป็น แบบปรนัย (Multiple Choice Questions – MCQs) มีตัวเลือก 5 ข้อ และระดับความยากตั้งแต่ พื้นฐานจนถึงขั้นสูง
โครงสร้างข้อสอบ & ตัวอย่างแนวข้อสอบ
1. กลศาสตร์ (Mechanics): กฎการเคลื่อนที่ของนิวตัน & แรงเสียดทาน
- คำนวณ แรงลัพธ์ และแรงเสียดทาน โดยใช้กฎการเคลื่อนที่ของนิวตัน
- เช่น วัตถุถูกดึงด้วยแรงทำมุมกับแนวราบ มีแรงเสียดทานกำหนดให้ คำนวณความเร่งของวัตถุ
2. งาน พลังงาน และกฎการอนุรักษ์พลังงาน (Work, Energy & Conservation Laws)
- หาค่างานที่แรงทำกับวัตถุ
- วิเคราะห์พลังงานศักย์และพลังงานจลน์
- เช่น วัตถุถูกยิงขึ้นฟ้า แล้วตกลงไปในทราย คำนวณงานที่แรงต้านจากทรายกระทำ
3. คลื่น และเสียง (Waves & Sound)
- วิเคราะห์ ฮาร์มอนิกของท่อเปิด-ปิด และความถี่ของเสียง
- เช่น หาความยาวของท่อเปิดปลายเดียวเมื่อกำหนดค่าความถี่ของฮาร์มอนิกที่สาม
4. แม่เหล็กไฟฟ้า และสนามแม่เหล็ก (Electromagnetism & Magnetic Field)
- คำนวณสนามแม่เหล็กจากกระแสไฟฟ้าโดยใช้ กฎของ Biot-Savart หรือ Ampere’s Law
- เช่น กระแสไฟฟ้าไหลในสายสองเส้นคู่ขนาน หาสนามแม่เหล็กที่จุดระยะหนึ่งจากสายไฟ
5. ฟิสิกส์อะตอม และโฟโตอิเล็กทริก (Atomic Physics & Photoelectric Effect)
- วิเคราะห์ การเปลี่ยนระดับพลังงานของอะตอม
- เช่น หาการเปลี่ยนพลังงานของอะตอมที่ดูดซับโฟตอนที่มีความยาวคลื่นยาวที่สุด
6. ระบบแรงตึงเชือก และการเคลื่อนที่แบบมีแรงกระทำ (Tension & Dynamics)
- วิเคราะห์ระบบวัตถุที่เชื่อมต่อกันด้วยเชือก และหาความตึงเชือก
- เช่น ระบบมวล 2 ก้อน ถูกดึงด้วยแรง F จงหาความตึงเชือกระหว่างวัตถุ
7. งาน และพลังงาน (Work & Energy Theorem)
- วิเคราะห์ว่าวัตถุถูกแรงดึงกระทำไปไกล D มีพลังงานศักย์หรือพลังงานจลน์เพิ่มขึ้นเท่าใด
8. การเคลื่อนที่ของวัตถุที่มีแรงเสียดทาน (Kinetic Friction & Motion)
- คำนวณระยะที่วัตถุเคลื่อนที่ก่อนหยุดจากพลังงานจลน์และแรงเสียดทาน
- เช่น บล็อกเคลื่อนที่ด้วยความเร็ว 4 m/s แล้วหยุดเนื่องจากแรงเสียดทาน 3 N คำนวณระยะที่บล็อกเคลื่อนที่ก่อนหยุด
9. การตกอย่างอิสระ และการเคลื่อนที่แบบโปรเจกไทล์ (Free Fall & Projectile Motion)
- ใช้กฎของการเคลื่อนที่แบบโปรเจกไทล์เพื่อคำนวณเวลาที่วัตถุตกถึงพื้น
- เช่น วัตถุถูกปล่อยจากความสูง 10 m คำนวณเวลาที่วัตถุตกถึงพื้น
10. การเคลื่อนที่แบบวงกลม (Uniform Circular Motion & Centripetal Force)
- วิเคราะห์แรงตึงเชือกในกรณีที่วัตถุเคลื่อนที่เป็นวงกลม
- เช่น หาความตึงของเชือกเมื่อรัศมีของวงกลม และมวลเพิ่มขึ้นเป็นสองเท่า
เทคนิคเตรียมสอบ CU ATS Physics
- ฝึกทำโจทย์คำนวณให้คล่อง – โดยเฉพาะหัวข้อ กลศาสตร์, งาน-พลังงาน, ไฟฟ้าแม่เหล็ก
- เข้าใจทฤษฎีหลักให้ดี – รู้ว่าแต่ละกฎของฟิสิกส์ใช้เมื่อใด
- จำสูตรสำคัญ
- ฝึกทำข้อสอบเก่า – CU-ATS Physics ปีเก่าๆ หรือ SAT Physics
- อ่านเนื้อหาให้ครบ – อย่ามองข้าม ไฟฟ้า, แม่เหล็ก, คลื่น, ฟิสิกส์อะตอม
- แนะนำหนังสือสำหรับเตรียมสอบ Fundamentals of Physics (Halliday & Resnick) , หนังสือ SAT Physics หรือ IB Physics HL















